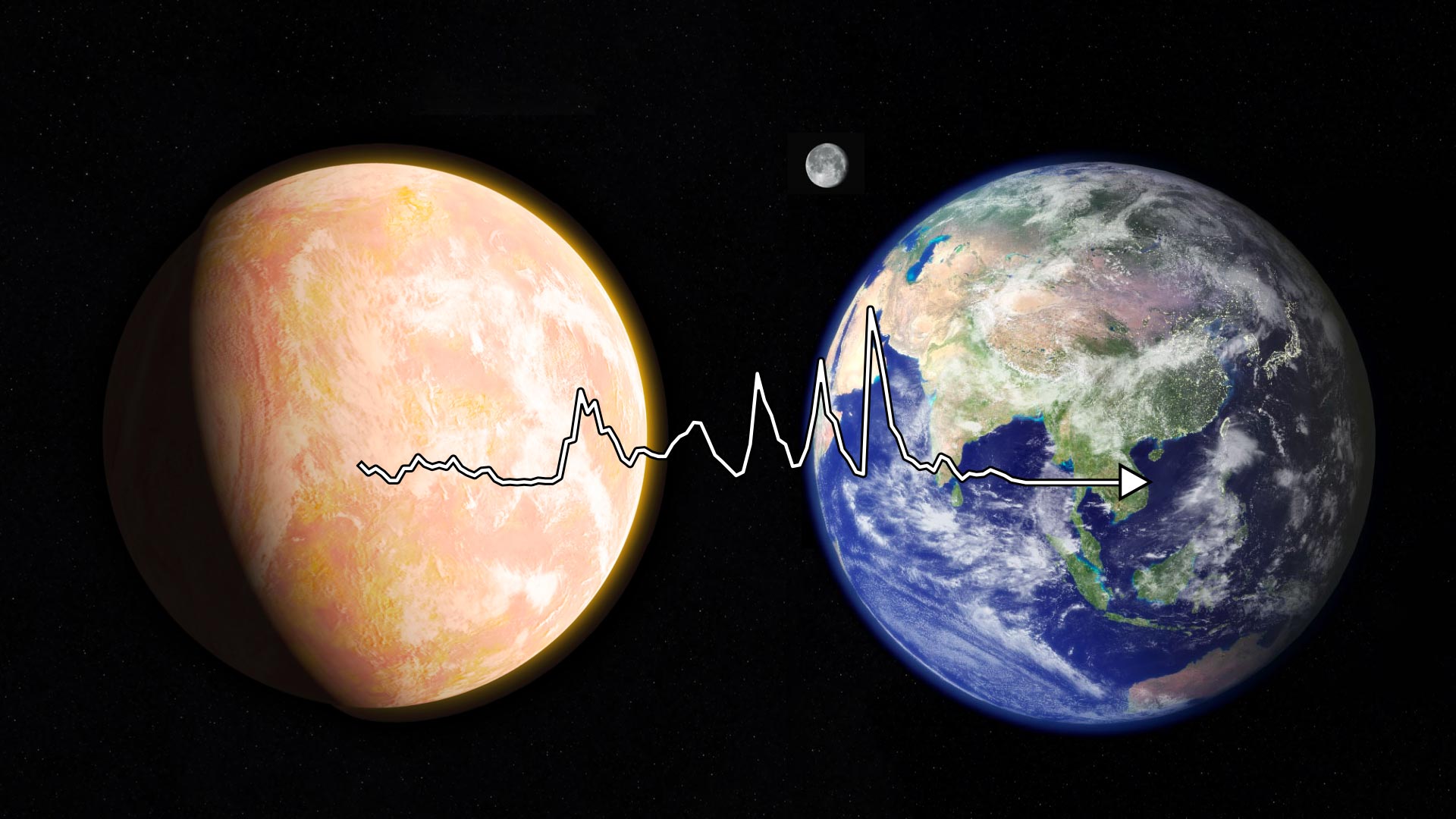
Metabolism is the “beating heart of the cell”. New research from ELSI traces the history of metabolism from early Earth to the present (left to right). The history of compound discovery over time (white line) is cyclical, almost resembling an EKG. Credit: NASA/Francis Reddy/NASA/ESA Goddard Space Flight Center
A new study shows that only a handful of “forgotten” biochemical reactions are needed to turn simple geochemical compounds into the complex molecules of life.
The origin of life on Earth has long been a mystery that eluded scientists. A key question is how much of the history of life on Earth is lost to time. It’s quite common for singles species to “phase out” by biochemical reaction, and if this occurs in enough species, life on Earth could effectively “forget” such reactions. But if the history of biochemistry is full of forgotten reactions, is there any way to tell?
This question inspired researchers from the Earth Science Institute (ELSI) at the Tokyo Institute of Technology and the California Institute of Technology (CalTech) in the US. They reasoned that the forgotten chemistry would appear as discontinuities or “breaks” in the path that chemistry takes from simple geochemical molecules to complex biological molecules.
The evolution of early Earth biochemistry
The early Earth was rich in simple compounds like hydrogen sulfide, ammonia, and carbon dioxide—molecules not typically associated with sustaining life. But billions of years ago, early life relied on these simple molecules as a source of raw material. As life evolved, biochemical processes gradually transformed these precursors into the compounds still found today. These processes represent the earliest metabolic pathways.
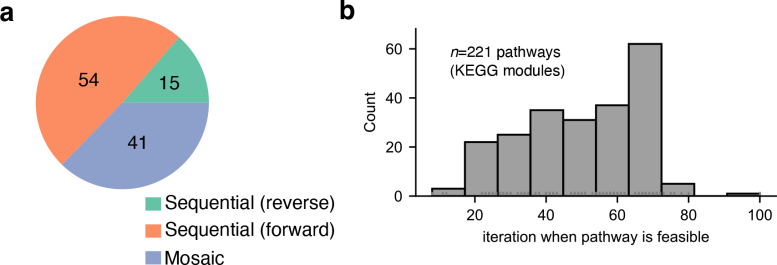
To build a model of the evolutionary history of metabolism at the biosphere scale, the research team compiled a database of 12,262 biochemical reactions from the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. Credit: Goldford, JE, Nat Ecol Evol (2024)
Biochemical Evolution Research Methodology
To model the history of biochemistry, the ELSI researchers—Specially Appointed Associate Professor Harrison B. Smith, Specially Appointed Associate Professor Liam M. Longo, and Associate Professor Shawn Erin McGlynn in collaboration with CalTech research scientist Joshua Goldford—needed an inventory of all known biochemical reactions to understand what types of chemistry life is capable of performing.
They turned to the Kyoto Encyclopedia of Genes and Genomes database, which cataloged more than 12,000 biochemical reactions. With the reactions in hand, they began to model the gradual evolution of metabolism.
Challenges in modeling metabolic evolution
Previous attempts to model the evolution of metabolism in this way have consistently failed to produce the most widespread complex molecules used by modern life. However, the reason was not entirely clear. As before, when the researchers ran their model, they found that only a few compounds could be produced.
One way around this problem is to nudge stalled chemistry by manually providing modern compounds. The researchers took a different approach: They wanted to determine how much reaction they were missing. And their hunt led them back to one of the most important molecules in all of biochemistry: adenosine triphosphate (ATP).
The ATP bottleneck and its solution
ATP is the cell’s energy currency because it can be used to drive reactions—such as making proteins—that would not otherwise occur in water. However, ATP has a unique property: The very reactions that make ATP require ATP. In other words, if ATP is not already present, there is no other way to make ATP for life today. This cyclical dependence was the reason why the model stopped.
How could this “ATP bottleneck” be solved? As it turns out, the reactive part of ATP is remarkably similar to the inorganic polyphosphate compound. By allowing ATP-generating reactions to use polyphosphate instead of ATP—by modifying a total of just eight reactions—almost all of the current core metabolism could be achieved. Scientists could then estimate the relative ages of all common metabolites and ask powerful questions about the history of metabolic pathways.
Metabolic pathways: linear vs. mosaic
One such question is whether biological pathways were created in a linear fashion—in which one reaction after another is gradually added—or whether pathway reactions emerged as a mosaic in which reactions of vastly different ages come together. to create something new. The researchers were able to quantify this and found that both types of pathways are almost equally common throughout metabolism.
Conclusion and implications
But back to the question that inspired the study—how much biochemistry is lost over time? “We may never know for sure, but our research has provided an important piece of evidence: only eight new reactions are needed to link geochemistry and biochemistry, all of which resemble common biochemical reactions,” says Smith.
“This does not prove that the space of missing biochemistry is small, but it does show that even reactions that have become extinct can be rediscovered from the traces left behind by modern biochemistry,” concludes Smith.
Reference: “Primitive Purine Biosynthesis Links Ancient Geochemistry to Modern Metabolism” by Joshua E. Goldford, Harrison B. Smith, Liam M. Long, Boswell A. Wing, and Shawn Erin McGlynn, March 22, 2024, Ecology and evolution of nature.
DOI: 10.1038/s41559-024-02361-4